1. Benign Epithelial Lesions
- Nonproliferative Breast Changes
- Proliferative Breast Disease Without Atypia
- Proliferative Breast Disease With Atypia
a. Nonproliferative Breast Changes (Fibrocystic changes)
Clinical features:
- Breast with many lumps and bumps on palpation
Radiological findings:
- Dense breast with multiple cysts
Morphology:
- Gross
- Blue dome cysts
- Microscopy
- Cystic change
- Apocrine metaplasia
- Fibrosis
- Adenosis
b. Proliferative Breast Disease Without Atypia
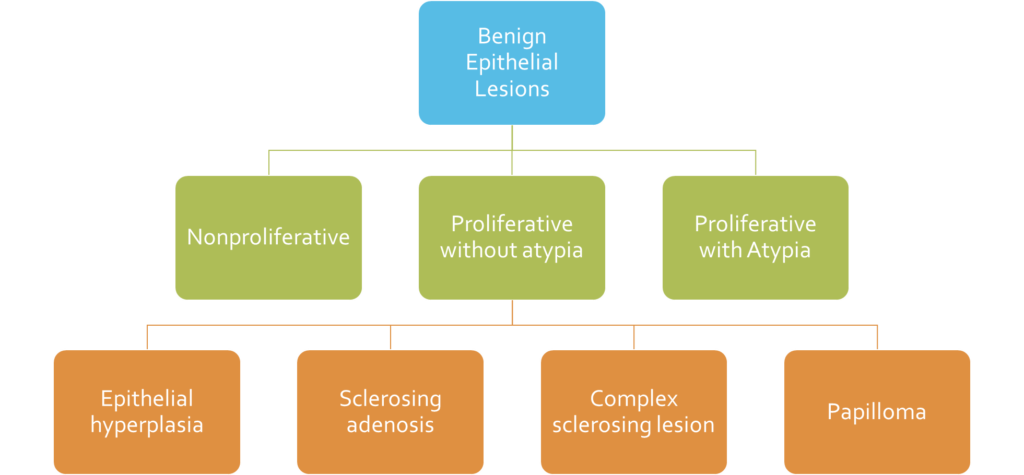
Epithelial hyperplasia
- Luminal(epithelial) and myoepithelial cells fill (and distend) ducts and lobules.
Sclerosing adenosis
- Sclerosing = Stromal fibrosis
- Adenosis = Increased number of acini
- Acini are compressed and distorted in the central part of the lesion
Papilloma
- Lesion within a dilated duct
- Multiple branching fibrovascular cores
- Epithelial hyperplasia + apocrine metaplasia may be present
- Nipple discharge – Bloody (infarction) or Serous (obstruction)
Complex sclerosing lesion
- aka radial sclerosing lesion or “radial scar”
- = sclerosing adenosis + papilloma + epithelial hyperplasia
Gynecomastia
- Enlargement of Male breast
- Most commonly see as Sub-areolar lump
- Etiology:
- Estrogen > Androgen
- Puberty
- Old age
- Cirrhosis etc
- Estrogen > Androgen
- Microscopy:
- Increased number of ducts
- Cellular stroma
- Usually lobule formation in absent
c. Proliferative Breast Disease With Atypia
- aka Atypical hyperplasia
- Clonal proliferation of epithelial cells
- but these lesions do not have all of the histologic features of carcinoma in situ (DCIS/LCIS)
Atypical ductal hyperplasia
- Has resemblance to DCIS.
- Monomorphic proliferation of regularly spaced cells
- Cribriform spaces.
- Only partially fills involved ducts (Difference from DCIS)
Atypical lobular hyperplasia
- Cells are identical to those of lobular carcinoma in situ (LCIS)
- But the cells do not fill or distend more than 50% of the acini within a lobule
2. Carcinoma in Situ
- Carcinoma in Situ = carcinoma in its original place
- Cancer cells are confined within ducts and lobules by a basement membrane.
- As there are no blood vessels or lymphatics within the basement membrane in a duct or acini, metastasis does not occur.
- Two types:
- Ductal Carcinoma in Situ (DCIS)
- Lobular Carcinoma in Situ (LCIS)
Ductal Carcinoma in Situ (DCIS)
- Clonal proliferation of epithelial cells
- limited to ducts and lobules by the basement membrane.
- Myoepithelial cells + (though reduced in number)
- Can spread throughout the ductal system
- Detected by mammography
- Morphology – different patterns
- Comedo DCIS
- High grade nuclei
- Central area of necrosis
- Cribriform DCIS
- round spaces (cookie cutter like)
- filled with calcified secretions
- Micropapillary DCIS
- Papillary projections without fibrovacular cores.
- Papillary DCIS
- true papillae with fibrovascular cores
- fibrovascular cores lack a myoepithelial cell layer
- Comedo DCIS
- Paget disease of the nipple
- Malignant cells from an underlying DCIS or malignancy in a breast travel through ducts to reach the skin of the nipple and destroy the nipple.
- Clinical feature
- Unilateral nipple itching with erosion, redness, oozing and scaling
- Pathogenesis
- Malignant cells from an area of DCIS travel through the duct system (without crossing the basement membrane) and reach the nipple skin
- When the malignant cells reach the squamous epithelium of nipple skin, they disrupt the barrier causing extracellular fluid to seep out and form an oozing scaly crust.
Lobular Carcinoma in Situ (LCIS)
- Clonal proliferation of cells within ducts and lobules that grow in a dyscohesive fashion.
- Usually not associated with calcifications or stromal reactions, so difficult to detect on mammography
- loss of cellular adhesion- E-cadherin (reason for dyscohesive growth)
3. CARCINOMA OF THE BREAST
Introduction
- Breast cancer is the most common cancer in women
- 1 in 8 women living up to 90 years will get breast cancer
- Social changes that have increased breast cancer risk
- delayed childbearing
- fewer pregnancies
- reduced breastfeeding
- lack of access to good health care
- Most common location: Upper outer quadrant of left breast
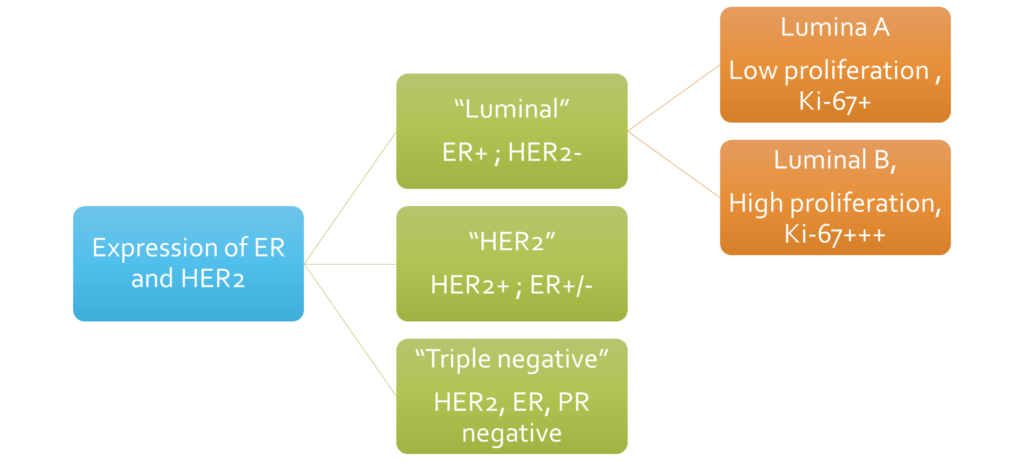
Molecular classification of invasive breast cancer
Pathogenesis
a. Familial Breast Cancer
- About one third of all breast cancers have a familial basis due to inheritance of susceptibility genes.
- Most common SINGLE gene mutations associated with susceptibility to Breast cancer:
- BRCA1 mutation
- Familial breast and ovarian cancer
- About 60% chance of getting breast cancer
- Triple negative Cancers
- BRCA2 mutation
- Familial breast and ovarian cancer
- About 30% chance of getting breast cancer
- ER positive cancers
- Other mutations
- p53
- PTEN
- CDH1
- BRCA1 mutation
b. Sporadic Breast Cancer
- More common than Familial ones
- About 2/3rd of all breast cancers
- Pathogenesis: Three major pathways (see molecular classification for comparison)
- Luminal (ER-positive/HER2-negative) cancers:
- Most common pathway – 50-60%
- Usually well differentiated tumours
- Slow growing
- Estrogen exposure is the major risk factor
- Pathway of luminal breast cancers:-
- Normal breast -> BRCA2 mutation/1q gain -> Flat epithelial atypia -> Atypical ductal hyperplasia (ADH) -> DCIS -> INVASIVE CANCER
- HER2 positive cancers (ER can be +/-):
- HER2 overexpression – chromosome 17q
- Pathway of luminal breast cancers:-
- Normal breast -> HER2 amplification -> DCIS -> INVASIVE CANCER
- Triple Negative Breast Cancer (ER -ve, PR -ve, HER2 -ve)
- Least common – 15%
- Basal cell like gene expression
- More aggressive
- Pathway of Triple Negative Breast Cancers:-
- Normal breast -> BRCA1/Tp53 mutation -> DCIS -> INVASIVE CANCER
- Luminal (ER-positive/HER2-negative) cancers:
Morphology
- Wide variety of morphologic appearances
- Most common pattern is Invasive breast cancer of no special type (IBC-NST), which is same as Ductal Carcinoma.
- About 1/3rd of the cancers are characterized as special types like Lobular carcinoma, Mucinous carcinoma etc.
Invasive breast cancer of no special type (IBC-NST) or Ductal Carcinoma
- Gross
- Hard, irregular, infiltrating, gray white mass
- Grating sound on cutting
- Microscopy
- Anaplastic duct lining cells
- Arranged in sheets, cords, nests, tubular pattern
- Abundant fibrous stroma
- Abundant atypical mitoses
- Nottingham grading (Based on Tubule formation, pleomorphism, mitosis, mnemonic-TNM)
- Well-differentiated (>70% tubules, rare mitotic figures)
- Moderately differentiated (greater degree of nuclear pleomorphism and contain mitotic figures)
- Poorly differentiated (enlarged irregular nuclei, Tumor necrosis are common, abundant mitosis, less tubules)
Special Histologic Types of Invasive Carcinoma
They have specific genetic aberrations, which cause specific histological patterns (which helps us in diagnosing these types) and have specific treatment and prognosis.
- Lobular Carcinoma
- Genetic aberration: loss of expression of CDH1 (the gene that encodes E-cadherin, which helps in cell to cell adhesion)
- Morphology:
- Dyscohesive cells (as E-cadherin is lost)
- Single cells infiltrating stroma as a line of cells, called “Indian file pattern”
- Minimal or No stromal response (desmoplasia) – so difficult to detect in mammography or by palpation.
- Signet ring cells
- Metastasis: Peritoneum, meninges, GIT etc.
- Prognosis: Good
- Carcinoma with Medullary pattern
- Genetic aberration: BRCA1 mutation
- Morphology:
- Soft (medulla=bone marrow)
- Minimal or No desmoplasia (that’s why its soft)
- Microscopy:
- Sheets of cells (poorly differentiated) like a syncytium
- High nuclear pleomorphism
- High mitosis
- Dense accumulation of inflammatory cells (T-lymphocytes, plasma cells) around the tumour sheets.
- Pushing border (Not infiltrating)
- Prognosis: Better than other poorly differentiated breast cancers.
- Mucinous Carcinoma (Colloid Carcinoma)
- Soft gelatin like
- Pushing border (Not infiltrating)
- Clusters of tumour cells floating in mucin (like islands in a lake)
- Prognosis: Very good
- Tubular Carcinoma
- Well formed tubules
- Apocrine change
- Calcification in lumen
- Can be mistaken for benign tubes
- Good prognosis
- Papillary Carcinoma
- Many papillae with fibrovascular cores
- Micropapillary carcinoma
- Papillae without fibrovascular cores
- Metaplastic carcinoma
- Differentiation of carcinoma towards squamous or spindle or chondroid or other mesenchymal elements.
- Inflammatory carcinoma
- Redness, thickening and swelling of skin of breast
- Can be confused with inflammation of breast
- peau d’orange appearance (orange like skin)
- Poorly differentiated
- Poor prognosis
Prognostic and Predictive factors of breast cancer
Prognostic factor = what will happen to the patient if no treatment is given, whether she/he will survive or not? eg: factors related to tumour extend or spread like tumour size, lymph node etc AND also factors related to tumour biology or behaviour like mitosis/proliferation, ER status etc.
Predictive factor = how will the patient respond to treatment, will survival improve after treatment? eg: factors related to tumour biology or behaviour like mitosis/proliferation, ER status etc. (note: these factors are both predictive and prognostic.)
Prognostic factors
- Histologic grade of cancer: high grade- poor prognosis, low grade- good prognosis
- Lymphovascular invasion: poor prognosis
- ER, PR, HER2 expression: ER+/PR+/HER2-ve has good prognosis, while triple negative has poor prognosis
- Special histologic types: Some types have better prognosis than others (see above in morphology)
- TNM
- T- Tumour size
- N- Lymph node status
- M- Metastasis
- Response to chemotherapy: Good response=good prognosis
- Gene expression profiling
Predictive factors
- ER, PR, HER2 expression
- Mitotic rate/proliferation – high=bad
- Grade
- Gene expression profiling
TNM Staging of breast cancer
- Stage 0
- T- Only DCIS
- N- Nil
- M- Nil
- Stage 1
- T- <2cm tumour size
- N- Nil
- M- Nil
- Stage 2
- T- 2-5cm tumour size
- N- 1 to 3 lymph nodes involved by tumour
- M- Nil
- Stage 3
- T- More than 5cm tumour size or tumour invading skin or chest wall
- N- 4 or more lymph nodes involved by tumour
- M- Nil
- Stage 4
- T- Anything
- N- Anything
- M- Metastasis present
4. STROMAL TUMORS OF THE BREAST
Intralobular stroma- Fibroadenoma (very common), Phyllodes tumour
Interlobular- Lipoma, Angiosarcoma (very rare)
Fibroadenoma
- Most common benign tumour of breast
- MED 12 mutation
- Biphasic- Contains both Stromal and Epithelial elements.
- Clinical features
- Age group: 20-40yrs
- Painless, firm, freely mobile lump breast
- Gross findings
- well-circumscribed
- rubbery, grayish white
- bulge above the surface when cut
- slit-like spaces
- Microscopy
- Proliferation of intralobular stroma, which pushes and compresses glands to slit like spaces (intracanalicular pattern)
- Glands may be surrounded by stroma (pericanalicular pattern)
- Slit like spaces and ducts are lined by Epithelial and Myoepithelial cells
- Prognosis: Very good
Phyllodes Tumor
- MED12 mutations
- Usually benign
- Biphasic- Contains both Stromal and Epithelial elements
- Clinical features
- Age group: 40-50yrs
- Painless, firm, mobile breast lump
- Can become extremely large, upto 20cm
- Gross findings
- Usually well-circumscribed (may become infiltrative in malignant cases)
- Leaf like projections into cyst like spaces.
- Microscopy
- Proliferation of intralobular stroma, with Leaf like projections covered by epithelium (exaggerated intracanalicular pattern)
- Low grade- similar to fibroadenomas, but with increased stromal cellularity
- Boderline- in between low and high grade.
- High grade- like sarcomas
- Prognosis: Good for low grade tumours.
